Technical Support技术支持
CONTACT US
 400 179 0116
400 179 0116
24-hour service hotline marketing@ldraft.comE-mail
marketing@ldraft.comE-mail
Highlight. - Necessary for cell knockout
source:QiDa technoligy views:10684 time:2023-04-23
Take the road less traveled and produce a knockout cell line instead of knockout cells? In this article, we give you tips and tricks for using homologous directed repair pathways, designing the best donor DNA, and avoiding common mishaps in such genome editing.
Knock-in mutation and gene editing
Targeted genome editing events fall into two broad categories: knockouts and knockouts. The purpose of knockout is to disrupt DNA translation by generating frameshift mutations. Most cell repair events produce these types of mutations, making it a "simple" editing job. However, this is not the case for the door knocker. Knock-in mutations usually require binding precise DNA sequences at precise genomic locations, leaving little room for error. Not surprisingly, these editors require more planning and skill than weeding out rivals.
To start a knocking experiment, the first question to ask is where to knock from, so you need to determine the location in the genome where you want to introduce your stepping stone. Then choose which Cas enzyme to use and design a gRNA where you want to introduce editing. Finally, you have to know what sequence to introduce, and this is done by designing and using the donor DNA molecule. Your donor molecule needs to be homologous to the target loci on either side of the new sequence. This homology will direct the cell to use the donor DNA as a template for repair, which will allow your sequence to be combined and eventually knocked out! So how is the donor being used as a template for restoration? See the breakdown below.
Homologous directed repair and genome engineering
The easiest way to create knockout cell lines is to use the built-in repair pathway that cells already have to repair DNA double-strand breaks -- (HR).
HR is the dominant homologous directed repair (HDR) pathway in mammalian cells, but is generally a less common DNA repair mechanism. On average, it accounts for less than 10 percent of DNA repair, and repair rates vary by cell type. The difference may be caused by cell cycle rate; Fast growing cells will have a higher HR frequency than slow growing cells. It is important to know that HR often mutates in cancer cells, especially in breast and ovarian cell lines commonly used in the laboratory. It is recommended that you check the cell line for HR-related mutations before attempting HDR knockout.
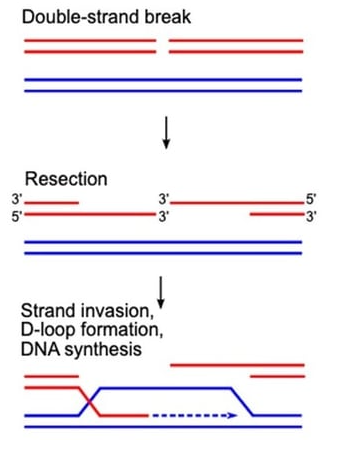
Figure 1: Early steps to repair a DNA double-strand break by homologous recombination.
Design HDR
The basis of the HDR pathway relies on the repair of template molecules, which are usually endogenously available sister chromatids, although foreign DNA can also be used. Here are some considerations for designing donor DNA for a successful HDR campaign.
CRISPR cutting position
Bring the CRISPR cut as close to the tapping position as possible. HDR relies on cutting ssDNA at the site of the break and using the ends of ssDNA to find the repair template. The highest HDR efficiency was observed when the insert was within 10bps of the break, making the cut essentially part of the template DNA.
Homologous sequence and sample type
To ensure that your donor molecule is used as a template, you need to add homologous sequences to the right and/or left of the knockin site. The length and composition of the sequence (single versus double) depend on the size of the vector. Single strand oligonucleotide donors (SSODNs) with homologous sequences up to 40bps can effectively tap into sequences of several hundred bps. However, if you have a larger knockout (200 bp -- 2 kb), dsDNA donors will need to be used due to oligonucleotide synthesis limitations. These vectors have traditionally had large homologous sequences in the 500bp to 1kb range, however, shorter homologous regions have been used recently with some success (Yu et al., Nat Chem Biol).
Mutate PAM or sgRNA sequences
Cas9 may undergo multiple rounds of cleavage at the target site until the PAM site or part of the guide recognition sequence is destroyed by mutation. By introducing a mutation into the PAM or guide recognition sequence of the donor DNA, HDR can be guaranteed not to be retargeted. If your Cas9 cuts into the coding region of a gene, make sure that the PAM edit you introduce is a silent mutation so that you don't accidentally change the protein you're interested in.

Figure 2: Stopping PAM recleavage and directing RNA destruction mutations in donor DNA
Elevates HDR above other edits
HDR is not the most common editing pathway for DNA repair. To tilt the scale during HDR editing, you can try one or more of the following different editing positions.
Other repair mechanisms
In addition to HR, there are two main repair mechanisms in mammalian cells -- non-homologous terminal junction (NHEJ) and alternative terminal junction (alt-EJ). Inhibitors of important components of both pathways have been shown to be effective in increasing HR editing success (Yang et al., Int J Mol Sci). Adding these inhibitors to the medium before and after Cas9 introduction will increase HDR, just be sure to remove the inhibitors within a day or two so as not to affect cell viability.
Enriched S phase cells
HR mainly acts on the S and G2 phases of the cell cycle, when sister chromatids may exist as repair templates. To maximize HDR release, ensure that the cell actively circulates during the cell cycle phase. If the cells are over-fused or don't have enough nutrients in the medium, they won't replicate their DNA, so be sure to keep an eye on these cells! Further, you can inhibit the cell cycle regulator responsible for the S-phase transition, allowing cells to stay in the HDR-promoted cycle longer. This approach may have some negative effects on viability if the cells stay too long. Prolonged cell cycle arrest can lead to apoptosis in as little as 10 hours.
Use fast Cas clips
What if advancing HR was as easy as replacing your Cas9? Yes! Cas12a is a member of the Cas family and produces sticky ends when cut. Cas12a may promote HDR by producing ssDNA suspensions at the site of the break, an early HR step (Moreno-Mateos et al., Nat-Com).
Another method used to initiate HR Cas cleavage is to fuse HR proteins to nucleases. Early HR factors fusing with Cas9 have been shown to increase the HDR frequency by interrupting the HR before other factors are loaded onto the break, potentially allowing the repair to proceed along a different pathway (Charpentier et al., Nat-Com).
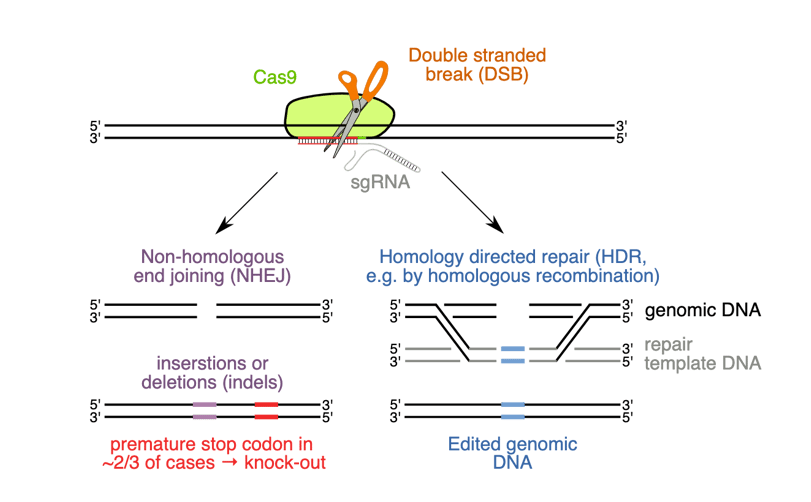
Figure 3:HDR cut competes with NHEJ mediated small insert and miss edits.
References
Yu, Y., Guo, Y., Tian, Qigi, Lan, Y., et. al. An efficient gene knock-in strategy using 5 '-modified dsDNA donors with short homology arms. Nat Chem Biol., 308(20): 1-9 (2020). 10.1038/s41589-019-0432-1. Doi: 10.1038/ S41589-019-0432-1
Mehdi Banan. Recent advances in CRISPR/Cas9-mediated knock-ins in mammalian cells. J Biotechnol., 16(4): 387-390 (2020). 10.1016 / j.j. Biotec 2019.11.010
Wright, D. W., Shah, S. S., Heyer, W. D. Homologous recombination and the repair of DNA double strand breaks. J Biol Chem., 293(27): 10524-10535 (2018).1 10.1074/ jpc.tm118.000372
Yang, H., Ren, S., Yu, S., Pan, H., et al. Methods favoring homology-directed repair choice in response to CRISPR/Cas9 Induced-double strand breaks. Int J Mol Sci., 21(18): 6461 (2020). 10.3390/ijms21186461
Lin, S., Staahl, B. T., Alla, R. K., Doudna, J. A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLIFE. (2014) 10.7554/eLife.04766
Moreno-Mateos, M. A., Fernandez, J. P., Rouet, R., Vejnar, C. E., et al. CRISPR-Cpf1 mediates efficient homology-directed repair and termperature-controlled genome editing. Nat. Com., 8(2024), (2017). 10.1038/s41467-017-01836-2
Charpentier, M., Khedher, A. H. Y., Menerot, S., Brion, A., et al. CtIP fusion to Cas9 enhances transgene integration by homology-dependent repair. Nat. Com., 9 -- 1133 (2018). 10.3390/ijms21186461以上翻译结果来自有道神经网络翻译(YNMT)· 通用场景







