Technical Support技术支持
CONTACT US
 400 179 0116
400 179 0116
24-hour service hotline marketing@ldraft.comE-mail
marketing@ldraft.comE-mail
Have you learned how to purify and cultivate primary tumor cells?
source:QiDa technoligy views:1349 time:2023-09-14
After the primary extraction of tumors, it is always found that there are mixed cells growing silently, which directly affects the experimental results. When using cultured cells in vitro for experimental research, in order to ensure the reliability, consistency, stability, and repeatability of the experimental results, it is required to use a single type of cell for the experiment, so as to conduct a series of studies on the function, morphology, and other changes of a certain cell, So we must purify the cells to maintain the consistency and verifiability of the experimental results.
Due to the irregularity and infinite growth of the tumor itself, cloning purification method is the preferred method for primary purification of tumors, which can be divided into capillary method, limited dilution method, plate cloning separation method, soft agar cloning separation method, single cell microscopy method, etc
1. Capillary method:
Dilute a certain amount of cell suspension (such as 1 * 10 ^ 5/mL or less) to 10 cells/mL, take 10 mL of diluted cell suspension, use several 0.5mm diameter and 8mm long capillary glass tubes (30-50 pieces), and under negative pressure, suck the suspension into each capillary tube. Under inverted microscope, check that each tube only enters one cell's capillary tube, and then place it in adaptive culture medium or a culture bottle (or plate) with feeder cells, Cultivated in a CO2 incubator, a cell propagates in a capillary tube and expands out of the tube, forming a single colony of cells.

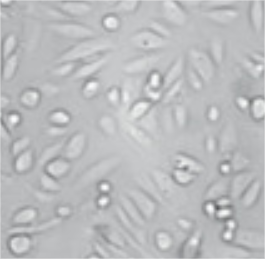
Primary culture of esophageal cancer cells Purified esophageal cancer cells
2. Limited dilution method:
(1) Take logarithmic growth stage cells and prepare a suspension (adherent cells digested with 0.25% trypsin and dispersed by blowing). Count them with trypan blue staining and measure the number and concentration of live cells (cell survival rate and individual cell percentage should be above 90%).
(2) Dilute the cell suspension in a test tube, and dilute the cells to 50 cells/mL, 10 cells/mL, and 5 cells/mL using culture medium. Inoculate cells of three different dilutions into a 96 well plate, with 0.1mL per well, and culture at 37 ℃ and 5% CO2.
(3) The next day, observe the number of cells in each well of the culture plate under an inverted microscope, select the well containing only one cell, mark it, and add 0.1mL of culture medium to continue cultivation.
(4) During the cultivation period, depending on the change in pH value, it is decided whether to change or supplement the culture medium. After about a week, there are obvious clones appearing in the well. When they reach 1/3 to 1/2 of the bottom of the well, digestion can be used to transfer the single clone cells from 96 wells to 24 well plates for expanded cultivation.
3. Plate cloning and separation method:
(1) Prepare a single cell suspension of logarithmic growth phase cells (suspension cells were dispersed by pipette blowing, adherent cells were first digested with 0.25% trypsin, and then dispersed by culture medium blowing) and count them. Adjust the cell concentration with culture medium to contain 50-200 cells in 5mL of culture medium (cell survival rate and single cell percentage should be greater than 90%).
(2) Quickly transfer the above cell suspension into a 60mm plate and incubate at 37 ℃ and 5% CO2 for 1 week or longer. If there is obvious clone formation, there are two methods for clone separation:
① Loop method: Observe the formation of clones under an inverted microscope, label the periphery of individual clones, dry the culture medium, use a sterile metal loop coated with a small amount of sterile silicone grease to wrap the labeled clones, drip a small amount of 0.25% trypsin into the loop, and when the cells detach, gently blow and disperse with an injector needle before transferring to a small plate, a 24 well plate, or a 6 well plate for expansion of culture.
② Glass slide method: Before inoculating cell suspension, sterile small glass slides are placed in a 60mm plate in advance, and the cell suspension is added. After a certain period of cultivation, the glass slide containing one clone is labeled under an inverted microscope. Then, sterile tweezers are used to remove the labeled glass slide and transfer it to a 24 well culture plate for further cultivation.
4. Soft agar clone separation method:
This method is only used for suspension cultured lymphoid like cells or highly malignant adherent cells, and easily digestible cells cannot form clones in soft agar.
(1) Prepare logarithmic growth stage cells into a single cell suspension (adherent cells were digested with 0.25% trypsin to disperse into individual cells) for live cell counting. Adjust cell concentration to 1 × 10 ^ 6 cells per liter, and then perform a gradient dilution according to the experimental requirements. Usually 1-5 × A liter of 10 ^ 4 cells is optimal.
(2) Prepare bottom layer agar. Take 5% agar and place it in boiling water to completely dissolve the agar. Take a portion of 5% agar and transfer it to a small beaker. Let it cool to 50 ℃. Quickly add 9 portions of fresh culture medium preheated at 37 ℃ (i.e. 0.5% agar), mix well, and immediately inject it into a 24 well culture plate. Each well contains 0.8mL of 0.5% agar, and set the agar at room temperature for later use.
(3) Prepare the upper layer of agar. Take 9.4mL of cell suspensions of different concentrations at 37 ℃ and transfer them to a small beaker. Add 0.6mL of 5% agar at 50 ℃ and quickly mix to form a 0.3% agar medium. Immediately pour it into a 24 well culture plate covered with the bottom layer of agar, with 0.8ml per well. Allow the agar to solidify at room temperature.
(4) Incubate at 37 ℃ and 5% CO2 for 1-2 weeks or longer. If a longer incubation time is needed, add 0.8mL/well of agar containing culture medium until there is significant colony formation.
(5) Colony (clone) counting: Observe and count under an inverted microscope with a diameter greater than 75 μ M or clones containing more than 50 cells.
5. Single cell micromanipulation method
With the help of a micromanipulator, individual cells are sucked out one by one under microscope monitoring and transferred to a culture plate containing feeder layer cells for cultivation. This method has good accuracy, and if there is no micromanipulator, a self-made capillary pipette can be used instead







