Technical Support技术支持
CONTACT US
 400 179 0116
400 179 0116
24-hour service hotline marketing@ldraft.comE-mail
marketing@ldraft.comE-mail
The protocol for HUVEC tube experiments is here
source:QiDa technoligy views:3794 time:2023-06-09
Angiogenesis involves not only pathological conditions including cancer biology and non-tumor diseases, but also many biological processes including reproduction, development and repair. During angiogenesis, endothelial cells (EC) are activated after angiogenic factors bind to their receptors, release proteases to dissolve the basement membrane, migrate to angiogenic signals, proliferate, and increase the number of cells to form new blood vessels. Finally, the recombination of endothelial cells forms a three-dimensional vascular system. The HUVEC tube formation assay is a simple but recognized in vitro angiogenesis assay based on the ability of endothelial cells to form three-dimensional capillary tubular structures when cultured on a growth faction-reduced basement membrane extract gel. During the assay, endothelial cells differentiate and migrate directionally to arrange, branch, and form a tubular polygon network of blood vessels.
Reagents to be prepared:
Matrix glue (Keida Bio, article No. :)
Dulbecco Modified Eagle Medium (DMEM) (High glycemic L-glutamine, 500 ml) (Keida Bio, item No. :)
Fetal bovine serum (Keida Bio, article No. :)
Primary human umbilical vein endothelial cells (HUVEC) (Keida Biology, product number: CD0290)
ECGM Endothelial Cell Culture Medium/Kit (Keida Bio, product No. : P1001)
ECGS (Keida Biology, Item No. : P1002)
Conditioned Medium (CM)
Note: Target cell lines can also be those with or without drug therapy or expressing genes of interest.
Supplies to be prepared:
Cell incubator
Inverted microscope with digital camera (Nikon TMS)
Scion Image software
96-well plate
centrifuge
Cell counter
T25 cell culture bottle
HUVEC tubular operation protocol:
A. Preparation of conditioned media from target cell lines
1. To prepare a conditioned medium (CM), inoculate target cells and grow to 30-40% confluent (depending on the growth rate of the cell line) with serum-free DMEM (e.g., 10ml for a T75 tissue culture vial; 2. Substitute growth medium for 24 hours, then harvest CM when cells have reached 60-80% confluent in T75 tissue culture vial.
If the CM is not used immediately after collection, a 0.5 ml CM bisected sample is taken and stored at -80°C.
B. Preparation of human umbilical vein endothelial cells (HUVEC)
1. Prepare the ECGM complete medium according to the requirement of medium ratio.
2. Inoculate HUVEC cells in T25 or T75 culture vials as needed to achieve 70-80% fusion.
3. Serum removal starved HUVEC cells for 3-6 hours (e.g., 5 ml for T25 tissue culture vials) prior to tube formation assay in the antibiotic-free medium 200PRF.
Note: At the end of serum starvation, proceed to procedure C step 1.
4. After coating the 96-well plate with growth factor reduced matrix gel, the cells were removed from the flask surface with trypsin, neutralized with serum with DMEM, centrifuged at 1200rpm (276xg) at room temperature for 3 min to precipitate the cells, and then resuspended in 2-3ml serum-free DMEM. HUVEC concentration was determined by counting cells. HUVEC cells were also re-suspended with 4×105/ml serum-free DMEM by suction up and down several times to ensure a uniform single-cell suspension.
Note: For matrix adhesives with reduced growth factors, the thawing/freezing cycle should be minimized.
5. Mix thoroughly while pipetting 500μl HUVEC cell suspension into 1.5ml test tube. Using a bench centrifuge, the cells were slowed down for 3 minutes at 4000rpm (1100rcf). The supernatant is carefully sucked out without interfering with cell precipitation. Remove as much of the supernatant as possible. Depending on the number of target cell lines to be used, an appropriate number of HUVEC cells are prepared in 1.5ml tubes.
Note: Each target cell line CM will suspend one tube of HUVEC and each suspended HUVEC will be allocated 3 triplicate holes. Thus, a total of 3 pores will be used for each target cell line.
6. Thaw 0.5ml of the target cell line CM in equal parts collected from step 2 of procedure a and replenish it with fetal bovine serum to a final concentration of 1% to reinsert HUVEC cell particles in step 5 of procedure B.
Note: Each target cell line should be in triplicate (100μl HUVEC cell suspension required per well).
C. The 96-well plate was coated with growth factor reduced matrix glue (Keida Bio, No.)
1. Thaw the appropriate volume of matrix glue at 4°C the day before use.
2. Pre-cool the 96-well plate and pipette tip at -20°C for 2-3 hours.
3. At the end of serum starvation of HUVEC cells, before counting HUVEC, the sample of 50μl matrix glue was equally divided into each well of the 96-well plate pre-cooled on ice. Rotate the planking until the gel is evenly distributed throughout the hole. It is very important to avoid the formation of bubbles. Allow it to polymerize on a horizontal surface at room temperature for 1 hour.
D. HUVEC cells were assigned to the coated 96-well plate
1. Completely mix the 100μl HUVEC cell suspension obtained in step 6 of procedure B into the labeled hole of the 96-well plate. The plates were cultured at 37°C and 5%CO2 for 4-6 hours.
2. Look at cells with a light microscope. Images of the capillary network were taken and the pipe length was calculated using Scion Image software.
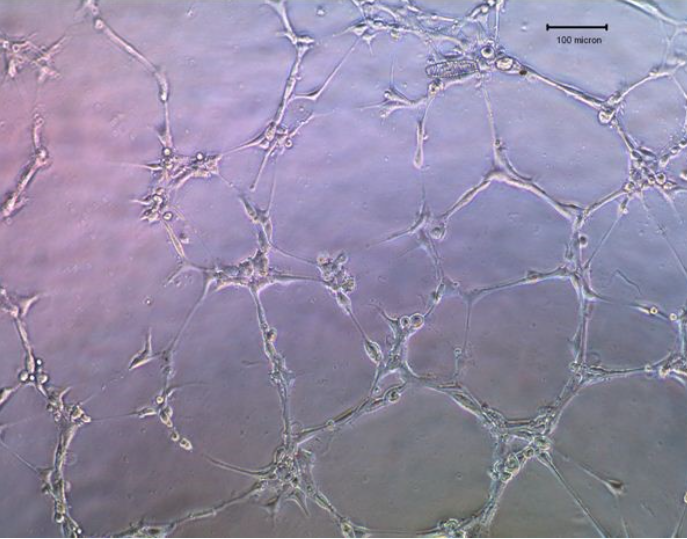
After incubation with CM for 6 hours (100x, 100μm), HUVEC was tubed on matrix gel.
References:
1. Ko, J. M. and Lung, M. L. (2012). In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay. Bio-protocol 2(18): DOI: 10.21769/BioProtoc.260.
2.Chan, K. C., Ko, J. M., Lung, H. L., Sedlacek, R., Zhang, Z. F., Luo, D. Z., Feng, Z. B., Chen, S., Chen, H., Chan, K. W., Tsao, S. W., Chua, D. T., Zabarovsky, E. R., Stanbridge, E. J. and Lung, M. L. (2011). Catalytic activity of Matrix metalloproteinase-19 is essential for tumor suppressor and anti-angiogenic activities in nasopharyngeal carcinoma. Int J Cancer 129(8): 1826-1837.
3.Kong, D., Li, Y., Wang, Z., Banerjee, S. and Sarkar, F. H. (2007). Inhibition of angiogenesis and invasion by 3,3'-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res 67(7): 3310-3319.







