Technical Support技术支持
CONTACT US
 400 179 0116
400 179 0116
24-hour service hotline marketing@ldraft.comE-mail
marketing@ldraft.comE-mail
Interpreting Literature: Organ like Culture and the Application of Biomedical Biology
source:QiDa technoligy views:2196 time:2023-05-26
This article is a literature interpretation, and the full text reading takes approximately 10 minutes
Organ like culture is an emerging 3D culture technology that has produced organoids from various organs and tissues, such as the brain, lungs, heart, liver, and kidneys. Compared with traditional two-dimensional culture, organoid culture systems have unique advantages in preserving parental gene expression and mutation characteristics, as well as maintaining parental cell function and biological characteristics for a long time in vitro. All these characteristics of organoids have opened up new opportunities for drug discovery, large-scale drug screening, and precision medicine. Another major application of organoids is disease modeling, especially for various genetic diseases that are difficult to model in vitro. Organoid modeling has been combined with genome editing techniques. Here, we introduce the development and latest progress in the field of organoid technology.
The study of organoids can be traced back to 1907, when Wilson et al. cultured mechanically dissociated sponge cells under in vitro conditions to form functional organisms. In the decades since then, organoid research has mainly focused on cell isolation and recombination. In 1975, a study cultured primary human keratinocytes and 3T3 fibroblasts to produce stratified squamous epithelial colonies similar to human epidermis. In the 1980s, the first batch of pluripotent stem cells (PSCs) were isolated from mouse embryos and obtained by humans. The development and progress of stem cell technology provided new ideas for the field of organoids. In 2009, intestinal adult stem cells (ASCs) were cultured in vitro to form small intestinal organoids with cryptic villous structures. This is a milestone event in the field of organoids, demonstrating the potential of stem cells to differentiate into organoid spatial structures in vivo. Since then, organoid culture technology has flourished and established organoids derived from various organs, such as the brain, retina, lungs, stomach, liver, bile ducts, pancreas, and kidneys.
The rapid development of three-dimensional (3D) culture technology plays a crucial role in achieving organoid culture. Cells in the body are located in complex internal environments and are influenced by various signals and interactions to establish, maintain, and regulate cell phenotypes and specific functions. The two-dimensional (2D) cultured cells failed to reproduce the normal cell morphology and interactions in vivo. The isolated histiocyte cultured in 2D system gradually lost their shape, became flat and abnormally divided, and affected the cell differentiation phenotype. 21, 22 2D attachment often leads to the loss of cells' original shape and hierarchical structure, and affects cell-cell and extracellular signal transduction and interactions, resulting in cells not being able to accurately reproduce the cellular functions and behaviors present in tissues or organs. The 23 2D tumor cell line gradually loses its heterogeneity during long-term cultivation. Meanwhile, long-term transmission is prone to cross contamination. It has been confirmed that there are significant differences in the genomics and metabolomics of cell lines compared to the original tumors after long-term passage. The 24 3D culture system can simulate the physical and chemical microenvironment of cells living in vivo and the interaction between cells and extracellular matrix. 25 Cells cultured in 3D system can better display complex structure and specific cell functions, highly match with parents, and maintain genetic stability and chromatin heterogeneity of parents. These cells can rapidly expand within 1-2 weeks and can be stably passaged and cryopreserved.
Organ like technology has paved the way for the development of new models that are closer to real-world physiological and pathophysiological states. In this review, we introduce the development and latest progress in the field of organoids, and describe the main cell sources and culture methods of organoids. We focused on the application of organoids in basic biology and clinical research, such as disease modeling and precise treatment, and emphasized their limitations and future prospects.
一、 The formation of organoids mainly includes the following categories:
PSC derived organs;
ASC derived organoids;
Tumor cell derived organoids;
Multilineage Organs
二、 Cultivation methods for organoids:
Immersion culture developed by the Clevers team is the most widely used culture method for organoids. In this scheme, cells or cell clusters are embedded in the extracellular matrix (ECM) gel, and then the mixture of cells and matrix gel is put into the culture dish to form a dome, and the culture medium is added to immerse the dome. ECM gel plays the role of structural support and providing ECM signal. Common ECM gel includes basement membrane extract, matrix and Geltrex. The main components of the culture medium used for immersion culture are composed of foundations, growth factors, stimulating factors, antibiotics, etc. When organoids are formed from different tissues, the composition of the culture medium varies, and which specific factors need to be added or removed usually depends on the corresponding signal or hormone requirements of the tissues from which they originate.
1. Ali system cultivation:
The 3D culture conditions for organoids can be achieved using the ALI technique first proposed by Ootani et al. 101 for establishing pancreatic and gastrointestinal organoids. The mechanically separated tissue fragments were evenly embedded in the collagen gel, the mixture was placed horizontally in an inner culture dish with a porous membrane below, and the top of the mixture was exposed to air. Add the culture medium to the outer culture dish. Kuo's team used the ALI method to establish tumor like bodies from various patient sources, including some rare tumor types such as ampullary adenocarcinoma of the bile duct. The 38 ALI culture system combines two culture dishes, which can simultaneously support the growth of organoid and stromal cells. It is worth noting that the organoid formed by this method can preserve functional tumor infiltrating lymphocyte (TIL), which can simulate the complex tumor immune environment.
2. Organ like chip culture system
Tissue on chip is a kind of microfluidic cell culture equipment, which can accurately control the biophysical and biochemical environment of cell growth, simulate the conditions of cells and microenvironment, as well as the interaction between tissues and multiple organs, for disease modeling and research on the functions of related organs;
Although there is a fundamental difference between organic matter on chips and organoids, the combination of organoid technology and organoids on chips can make up for the shortcomings of these two technologies and serve as a more effective preclinical model for simulating key features of target organ tissue. The organ cells on the chip are randomly and spontaneously self-organized into 3D structures, which is different from the carefully designed tissues on the chip. Currently, many studies have identified on-chip organs in the brain, heart, gastrointestinal tract, liver, and pancreas. Cho et al. 110 developed a brain chip based organ system based on PDMS chips, which can increase oxygen supply and promote nutrient/waste exchange to reduce cell death in organoid cells. It is worth noting that this culture system can form mature brain like organs and be used to monitor the development of the entire human brain. A recent study has shown that organoid systems on chips can generate hypoxia gradients in the lumens of small intestinal organoids and maintain the intestinal microbiota barrier.
In addition to the above cultivation methods, 3D systems of organoids can also be prepared through suspension and rotation cultivation methods. The suspension method utilizes gravity and surface tension to suspend a mixture of cells and specific culture medium droplets on a flat plate. 118 The rotating culture method can prevent cell sedimentation by constantly rotating or stirring cells, and improve the absorption of nutrition and oxygen, which has been used for the formation of brain and retinal organs. 120 Jacob et al121 produced glioblastoma like organs from patients, retaining the histological and genetic characteristics as well as some microvessels and immune microenvironment.
Four biomedical applications of organoids
1. Constructing Organs for Genetic Diseases
Organs can simulate organ development in vitro and can be used to model and study the mechanisms of organ specific genetic diseases; For example, liver like organs are composed of α 1- Produced by patients with antitrypsin deficiency, it was found that A1AT protein aggregates in organoid cells, which may represent α 1. Key pathological and pathophysiological characteristics of antiproteinase deficiency. Other hereditary diseases of the liver and gallbladder, including Alagille syndrome, Wilson's disease, and Wolman's disease, have been modeled using liver and gallbladder like organs. Islet like organs have been used to simulate genetic diseases related to insulin secretion, such as Wolfram syndrome and congenital hyperinsulinemia.
Kim et al. edited iPSC derived kidney like organs using CRISPR-Cas9 technology to generate a Fabry nephropathy model with GLA mutations. Its application range ranges from studying pathophysiology to exploring new treatment options for Fabry nephropathy. Prepare cardiac organs derived from HAND1 or NKX2-5 knockout iPSC system to simulate hypoplastic left heart syndrome. Congenital heart defects induced by diabetes before pregnancy can also be simulated by human cardiac organs; Organ genetic diseases derived from neuroectoderm can also be modeled by organoid; Retinal organs have been used as powerful tools for modeling and studying the mechanisms of hereditary retinal diseases such as retinitis pigmentosa and Leber's congenital amaurosis.
2. Constructing Organs for Infectious Diseases and Diseases
Since infectious agents usually infect specific species or cell types, human models are superior to animal models in studying the pathogenesis and development of these diseases. Ettayebi et al. first successfully cultured human norovirus in vitro with epithelial monolayers derived from human intestinal organs, and they optimized the culture system to enable it to cultivate a variety of strains. A culture system for influenza virus, human rotavirus, and BK virus has been established using organoids;
Despite progress in treatment, liver diseases caused by viral infections remain a national health issue in China. When the hepatitis virus invades and attacks liver cells, the immune response is activated, killing the virus and virus infected liver cells, causing degeneration and necrosis of liver parenchymal cells, accompanied by reactive matrix tissue proliferation, leading to the accumulation of collagen fibers in the liver. Human liver like organs have become advanced tools for studying hepatitis virus infection, and several laboratories have produced liver like organs infected with hepatitis B and C, as well as E virus. Recent studies have shown that when liver organoids from healthy donors are co cultured with the serum of recombinant virus or HBV patients, the organoids are infected and the virus activity increases.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogen of coronavirus disease (COVID-19) in 2019, which usually leads to moderate respiratory diseases such as fever, cough, and even acute respiratory syndrome. At present, alveoli, lungs, airways and bronchi have been generated to simulate SARS CoV-2 infection in vivo and study the pathophysiology related to COVID-19. In addition, COVID-19 also leads to extrapulmonary diseases, including gastrointestinal and neurological symptoms, as well as liver and kidney damage. The corresponding organics have been applied to SARS CoV-2 related research.
The application of organoid models in infection biology is not limited to virus research, but can also be used to study bacteria and protozoan parasites. Human small intestine and lung like organs infected with Cryptosporidium have been established. Similarly, the relationship between the blood-brain barrier and Plasmodium falciparum and Lyme's neuropathy was explored through blood-brain barrier like organs. A recent study showed that Hp infection can activate NF- κ B signaling pathway and upregulation of RAS protein activator like 2 (RASAL2) expression to promote the proliferation of gastric tumor cells. The downregulation of RASAL2 can significantly inhibit the growth of gastric tumors. The gallbladder like organs of mice infected with Salmonella typhimurium 185 contribute to malignant transformation (TP53 mutation and c-MYC amplification) and activate AKT and MAPK signaling pathways, thereby promoting abnormal cell proliferation and tumor transformation.
In summary, the 3D organ like model of infectious biology can reflect the correlation and interaction between pathogenic microorganisms and host cells, providing a key preclinical model for exploring the mechanisms, treatment, and drug development of infectious diseases. In addition, the establishment of a co culture system for organoids and carcinogenic pathogens provides an advanced platform for further research on the mechanisms of biological pathogens promoting tumor formation.
3. Building metabolic disease like organs
Currently, metabolic diseases pose a serious threat to health worldwide. However, the lack of suitable models limits the exploration of potential mechanisms and treatment methods. Organ like culture technology provides new ideas for research in this field.
Obesity is a common metabolic disease characterized by increased adipose tissue and increased risk of type 2 diabetes and NAFLD. Adipose organs have been used to study fat related metabolism and obesity models.
Alcoholic liver disease (ALD) is one of the most common chronic liver diseases worldwide. The initial stage of ALD is alcoholic fatty liver, which is characterized by steatosis of liver cells. Some patients may develop into alcoholic steatohepatitis, liver fibrosis, and cirrhosis, ultimately leading to cancer and liver failure. The ALD organ model 196 reported by Wang et al. was generated by co culturing human fetal liver mesenchymal cells/human ESC derived expandable liver organs with ethanol. This model can simulate typical pathophysiological features of ALD, including increased secretion of alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase; Decreased cell viability and apoptosis; Increased activity of CYP2E and CYP3A4; Enhanced oxidative stress; Increased release of inflammatory cytokines; Fibrosis; And an increase in ECM deposition.
4. Construction of tumor like organs
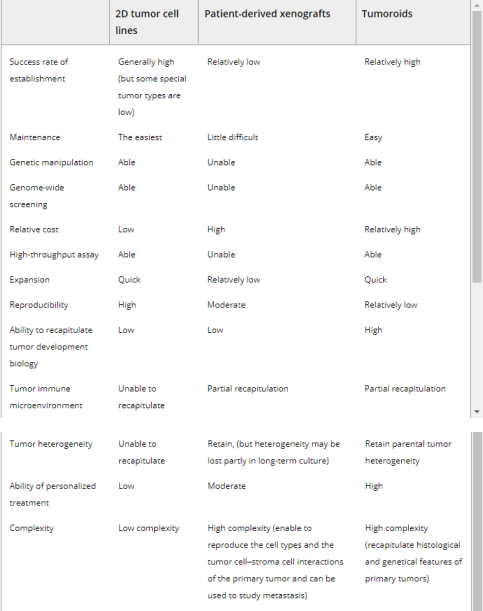
At present, the most commonly used oncology models mainly include human tumor cell lines and patient derived xenograft (PDX) models. However, these models have some inevitable drawbacks. The tumor cell line includes primary (derived from patients) and immortalized tumor cells. Although primary cancer cells retain some characteristics of the mother tumor, their slow growth rate, short lifespan, and lack of tumor complexity limit their application. Immortalized cells have unlimited proliferative capacity, but during long-term cultivation and passage, they typically cannot represent the phenotype of the original tumor and lose genetic heterogeneity, which may increase the failure rate of clinical drug screening trials. The 197 2D culture system cannot simulate in vivo cell growth conditions, and 2D cultures cannot accurately represent the heterogeneity of tumors. The PDX model is constructed by transplanting human tumor cells into mice and promoting their growth and tumor formation. The xenograft model can maintain the relatively complete biological characteristics, 3D structure, and tumor stroma of the parent tumor. Research has successfully generated several tumor PDX models, such as lung tumors, colorectal cancer, pancreatic tumors, breast tumors, prostate tumors, and ovarian tumors. However, some shortcomings limit their ability to become excellent preclinical models. Xenotransplantation models are usually established from a small number of tumor tissues that cannot fully inherit gene mutations from the primary tumor, and as PDX grows, the stroma of human tumors is gradually replaced by mouse stroma. Many research results based on the PDX model have not been confirmed in human experiments. In addition, the PDX model has higher economic and time costs, but lower success rates. Class 24 tumors retained the heterogeneity and histopathology characteristics of their parent tumors, and retained their 3D structure 3233205-207 (Table 1) after long-term culture. Papaccio et al. 208 reported that colorectal tumor like tumors from patients represent the morphological and immunohistochemical characteristics as well as genomic and transcriptome characteristics of corresponding tissues, and these tumor like drugs from different patients have different responses to anticancer drugs.
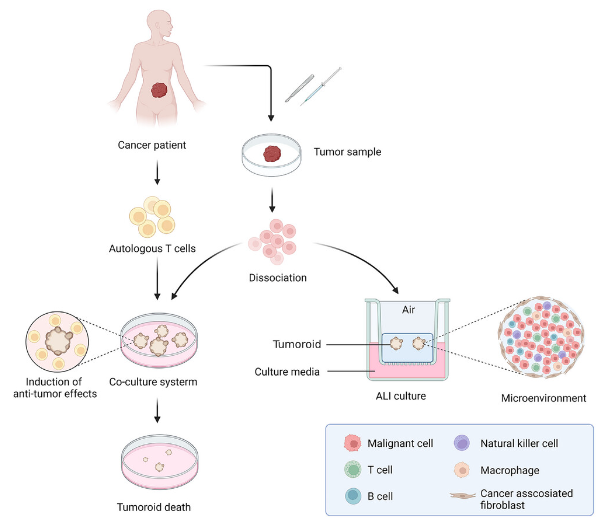
Remodeling tumor immune microenvironment with tumor like organs. Tumor samples are obtained through resection or biopsy and dissociated into individual cells to form tumor like organs. Tumor like organs are established by tumor cells and co cultured with autologous T cells through immersion, which can induce specific anti-tumor effects of T cells, leading to tumor like injury and death. With the application of ALI method, tumor like organs are used as initial cell materials to generate tumor like cells that preserve cancer related fibroblasts and immune cells, which can reproduce complex tumor microenvironment in vitro (created using BioRender. com)
One potential application of organic compounds in precision medicine is the development of personalized cancer treatment. PDO can be used to test the efficacy of different chemotherapy drugs and determine the most effective treatment method for this patient. An article by Khan et al. describes the application of organoids in predicting the response of patients with metastatic tumors of the digestive system to various chemotherapy drugs and targeted drugs.
Due to the important role of organoids in targeted therapy theory, organoids are also used for screening targeted drugs and identifying potential therapeutic targets. Ovarian tumor like organs from patients with three tumor types have been used as a high-throughput screening platform by Phan et al. to test the sensitivity to 240 protein kinase inhibitors.
Finally, organoids can be used to study the effects of environmental toxins and other factors on human tissues. By exposing organoids to different environmental conditions, researchers can study the effects of air pollution, toxic chemicals, and other factors on human health and determine personalized strategies to reduce exposure and prevent diseases.







