Technical Support技术支持
CONTACT US
 400 179 0116
400 179 0116
24-hour service hotline marketing@ldraft.comE-mail
marketing@ldraft.comE-mail
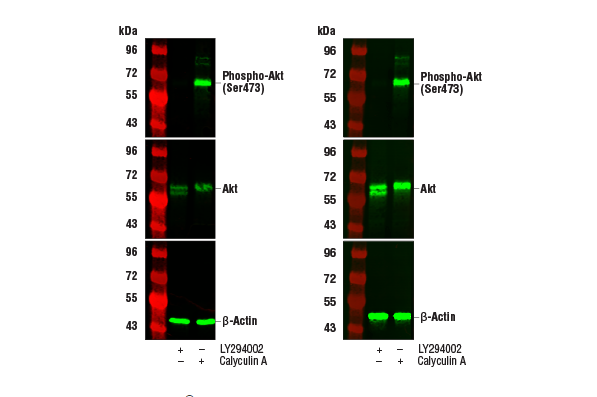
Experimental Steps for Protein Imprinting (Fluorescence)
source:QiDa technoligy views:1649 time:2023-04-03
A. Solutions and reagents
Note: Use reverse osmosis deionized water (RODI) or equivalent grade water to prepare the solution.
Prepare 1X phosphate buffer (PBS), 1X Tris salt buffer (TBS), and 1X SDS sample buffer:
A fresh 3X reduction loading buffer was prepared by adding 1/10 volume of 30XDTT to 1 volume of 3XSDS loading buffer. Dilute to 1X with dH2O.
10X Tris Glycine SDS Electrophoresis Buffer: To prepare 1L1X
Electrophoresis buffer: Add 100ml of 10X electrophoresis buffer to 900ml dH2O, and then mix well.
10X Tris-Glycine transfer buffer: To prepare 1L1X transfer buffer, add 100ml of 10X transfer buffer to 200ml of methanol+700ml of dH2O, and then mix well.
10X Tris salt buffer containing Tween20 (TBST-10X): To prepare 1L 1XTBST, add 100ml of 10X TBST to 900mldH2O, and then mix well.
non-fat milk powder:
Blocking buffer: 1XTBS containing 5% w/v skimmed milk powder; To prepare 150 ml, add 7.5 g of skimmed milk powder to 150 ml of 1X TBS and mix well. Tween20 should not be added to a closed buffer because it has spontaneous fluorescence and can add a nonspecific background. After the sealing step, Tween20 is added to the subsequent diluent buffer.
Washing buffer: 1XTBST.
Bovine Serum Albumin (BSA)
First antibody dilution buffer: 1X TBST containing 5% BSA or 5% skimmed milk powder, as shown on the first antibody data sheet; To prepare 20ml, add 1.0g BSA or skimmed milk powder to 20ml of 1X TBST and mix well.
Second anti dilution buffer: 1XTBST containing 5% skimmed milk powder; To prepare 20ml, add 1.0g of skimmed milk powder to 20ml of 1X TBST and mix well. (Second anti selection; anti rabbit or anti mouse).
Pre-stained protein standard, wide range (11-190kDa):.
Imprinting film and paper: Nitrocellulose film, typically recommended with a pore diameter of 0.2 µ m.

B. Protein imprinting
Routine process for preparing samples.
1. Add fresh culture medium containing regulatory factors to treat the cells for a period of time.
2. Extract the culture medium from the culture; Wash cells using cold 1XPBS; draw.
3. Add 1X SDS sample buffer (100 µ l per well for 6-well plates or 500 µ l per plate for 10cm diameter plates) to lyse cells. Immediately scrape the cells off the plate and transfer the extract to a microcentrifuge tube. Place on ice.
Ultrasound treatment for 10 – 15 seconds to complete cell lysis and cut DNA (to reduce sample viscosity).
4. Take 20 µ l of sample and heat it at 95 – 100 ° C for 5 minutes; Cool on ice.
5. Centrifuge in a micro centrifuge for 5 minutes.
6. Sample 20 µ l onto SDS-PAGE gel (10cm x 10cm).
Note: It is recommended to sample a pre stained protein molecular weight standard (10 µ l/lane) to verify electrical transfer and determine molecular weight. The pre dyed standard has spontaneous fluorescence in the near infrared wavelength range.
7. Electrically switch to nitrocellulose membrane.

C. Membrane blocking and antibody incubation
Note: The capacity is suitable for 10cm x 10cm (100cm2) membranes; For membranes of different sizes, please adjust the capacity accordingly. After transfer, wash the nitrocellulose membrane with 25ml TBS at room temperature for 5 minutes.
The membrane was placed in 25 ml of closed buffer and incubated at room temperature for 1 hour.
Key step: Do not add Tween 20 to the closed buffer.
8. Wash with 15ml TBST three times for 5 minutes each time.
9. Incubate the membrane and primary antibody (diluted to a relative concentration) in 10 ml of primary antibody dilution buffer at 4 ° C overnight and gently shake them when necessary.
Wash with 15 ml TBST three times for 5 minutes each time.
The membrane and fluorescein coupled secondary antibody (1 mg/ml stock solution with a dilution of 1:5000-1:25000) were incubated at room temperature in 10 ml of secondary antibody dilution buffer for 1 hour, and gently shaken from time to time.
Wash with 15 ml TBST three times for 5 minutes each time.

D. Protein testing
Drain the excess TBST on the membrane to allow it to dry.
Key step: For fluorescent staining, it is necessary to ensure that the film is dry.
Please use a suitable fluorescent scanner to scan the film.