Technical Support技术支持
CONTACT US
 400 179 0116
400 179 0116
24-hour service hotline marketing@ldraft.comE-mail
marketing@ldraft.comE-mail
Worth collecting, steps for staining similar organs
source:QiDa technoligy views:1571 time:2023-12-21
How to stain a well made organoid so that it not only looks beautiful but also meets the requirements of your experiment? Follow the steps below:
Freezing and embedding:
1. Cut off the tip of P200ul and transfer the organoid into a 1.5ml Eppendorf tube;
2. Take out the culture medium and wash twice with 1ml PBS;
3. Fix in cold 4% PFA for 30 minutes on a shaking table at 4 degrees Celsius;
4.Wash three times with 1ml PBS;
5. Add 1ml of 30% sucrose to the test tube and incubate at 4C for 1 hour or until the organoid precipitates to the bottom;
6.During the cultivation process, use double-layer aluminum foil to prepare molds;
7. Preparation of ethanol: dry ice slurry;
8. Remove sucrose and wash once with 1ml PBS;
9. Cut off the tip of P200. Set the P200 pipette to 100ul;
10. Apply FBS to the tip of P200. Pick up all the organoids at once and let them settle to the bottom of the tip. Gently press down on the pipette throttle valve until the organoid gathers droplets at the bottom of the cutting tip;
11. Gently touch the droplets onto the bottom of the aluminum foil mold. Organs should stick together, and you should minimize the amount of PBS entering the mold. If there is too much PBS, please use P200 to carefully remove excess PBS;
12. Cut off the tip of P1000. Add 900ul of OCT/30% sucrose mixture (2 parts OCT/1 part 30% sucrose) to an aluminum foil mold containing organoids;
13. Use the P200 tip to rotate the organic compounds in the OCT/30% sucrose mixture. Dilute PBS and separate organoids from each other;
14. Place the mold in ethanol: dry ice slurry to ensure that ethanol does not enter the mold. OCT should be frozen and turn bright white within two minutes;
15. Place the mold in a 24 well plate and store it at -80 degrees Celsius until sliced. Once sliced, you can fix PFA below 5.
Immunostaining preparation:
1. Use a PAP pen to outline the contours of each part. Let it dry for 1-2 minutes;
2. Fix and slice with 4% PFA in a glass chamber at room temperature for 5 minutes;
3. Wash 3 times under RT with 1X PBS in a glass chamber for 5 minutes;
4. At room temperature, seal/permeate in a humidification chamber for 1 hour, and the sealing solution formula is as follows:
a. 10% inactivated horse serum in 50ml PBS
b. 0.38g glycine
c. 150ul Triton X-100
d. 1 drop of Image-IT signal enhancer
5. Add the first antibody to 1% inactivated horse serum in PBS. Incubate at room temperature for 1 hour or shake at 4C;
6.Wash in a glass chamber of 1X PBS+0.05% TritonX-100 for 3x5 minutes; (a. 100 ml PBS, b. 500uL 10% TritonX-100)
7. Add secondary antibodies to 1% inactivated horse serum+1:1000 DAPI and oscillate under RT for 2-3 hours;
8.Wash in a glass chamber of 1X PBS+0.05% TritonX-100 for 3x5 minutes;
9.Wash in 1X PBS for 2 to 5 minutes;
10. Take it out for brief drying. Add 50-100uL of Fluoromount to each type of organic compound and add a cover glass. Seal with transparent nail polish.
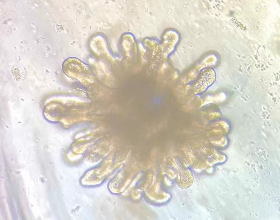
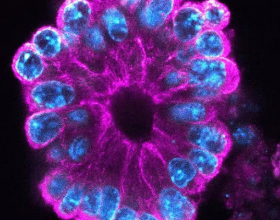

Start staining:
1. Remove cells from the incubator and gently wash once with 1X PBS (150ul for 96 well plates, 500ul for 24 well plates). If the cells are not firmly attached to the culture dish, manually aspirate instead of using vacuum;
2. Add 4% PFA to each well (50ul for 96 well plates and 400ul for 24 well plates) and incubate at room temperature for 15 minutes;
3. Add 1X PBS (150ul for 96 well plates and 1ml for 24 well plates) to each well to dilute PFA. Then clean three times and completely remove PFA with 1X PBS;
4. Add 0.1% Triton (50 μ Used for 96 well plates, 400 μ (Used for 24 well plates) to allow cell permeabilization and incubate for 10 minutes;
5. Remove 0.1% Triton and add a closed solution (10% normal donkey serum diluted in 1X PBS; 50ul for 96 well plate, 400ul for 24 well plate) and stir at room temperature for 1 hour;
6. Take out the sealed solution and add the primary antibody diluted in the sealed solution (30ul for 96 well plates, 300ul for 24 well plates). Incubate overnight at 4 degrees Celsius;
7. Wash three times with 1X PBS wells (150ul for 96 well plates and 1ml for 24 well plates);
8. Add the second antibody diluted in a closed solution and incubate at room temperature in the dark for 1-2 hours;
9. Wash once with 1X PBS (150ul for 96 well plates and 1ml for 24 well plates);
10. Add DAPI solution (1:10000 in 1X PBS; 50ul for 96 well plate, 400ul for 24 well plate) and incubate in the dark for 5 minutes;
11. Wash twice with 1X PBS wells (150ul for 96 well plates and 1ml for 24 well plates);
12.The sample can now be imaged. If the cells are on a cover glass, they can now be placed under a sliding lens for photography.







