Technical Support技术支持
CONTACT US
 400 179 0116
400 179 0116
24-hour service hotline marketing@ldraft.comE-mail
marketing@ldraft.comE-mail
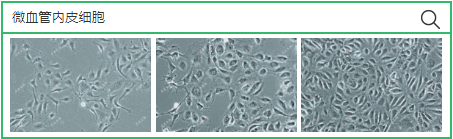
Extraction methods and magnetic bead screening methods suitable for various microvascular endothelial cells
source:QiDa technoligy views:2096 time:2023-07-27
How to extract and separate the microvascular endothelium of mice? Here is the information we have compiled through our technology. Based on the experience of 15 mice, do you have a better operating method? Welcome to leave comments on the backend and discuss together
Prepare the ECGM endothelial cell culture medium (in order to prevent pollution, the triple antibody can be used for primary culture) (Qida Biological: P1001), cell coating solution (Qida Biological: SD0044), PBS containing 2.5% FCS plus triple antibody, magnetic bead antibody, cell sieve, trypsin, ophthalmic scissors, etc

1. Using excessive amounts of isoflurane to euthanize mice. It is better to stop breathing in one minute. If the time is too long, there will be ischemic perfusion reaction, which will lead to too many blood cells in the extracted cells, and normal endothelium cannot obtain Vegetative reproduction.
2. Use high-pressure sterilization equipment to remove the heart, lungs, and liver of mice as sterile as possible, and rinse in PBS with 2X double antibody to remove blood.
3. In a Petri dish, use a sterile cross Scalpel to dissect the tissue to be extracted into 2mm ³ Block.
4. Wash twice in PBS using low-speed centrifugation (210 x g, 1 minute).
5. In a wet incubator at 37 ° C, culture the sectioned tissue in Collagenase 2 solution (0.5 mg/ml) for 1 hour.
Note: We use Gibco ® Type II Collagenase, because it has higher Clostripain activity than other Collagenase preparations, is very suitable for digesting heart, bone, thyroid, cartilage and liver tissues.
6. Subsequently, 75 µ l DNase I solution was added to every 10 ml of cell suspension and stirred continuously in a 37 ° C water bath for 30 minutes.
7. Pass the digested tissue through a cell filter to remove undigested chunks.
8. Rinse the cell filter twice with PBS supplemented with 2.5% FCS and dual antibody to collect any remaining cells.
9. Incubate in 0.25% trypsin (1ml trypsin per 100mg of tissue) for another 10 minutes to obtain a single cell suspension.
10. Wash once in 500 µ l PBS supplemented with 2.5% FCS.
11. Incubate with mouse immunoglobulin at 4 ° C for 30 minutes to block Fc receptor.
Note: The mouse BD-Fc block is a purified rat IgG2b anti mouse CD16/CD32 monoclonal antibody.
12.Wash twice in cold PBS supplemented with 2.5% FCS.
13. Incubate with rat anti mouse CD31, rat anti mouse CD105 and Biotinylation isolating protein B4 at 4 ° C for 45 minutes.
14. Wash twice in cold 500 µ l PBS supplemented with 2.5% FCS and count cells.
15. Resuspended particles and combined with PBS 2.5% FCS (200 μ L/l, 2.5 x 107 cells), rat anti mouse Ig (25 μ L/l, 2.5 x 107 cells) and streptavidin bound microspheres (25 μ L/l, 2.5 × Incubate 107 cells at 4 ° C for 15 minutes (total volume 250 μ l) . At the same time, load the chromatographic column onto the separation device (one column per 1-2.5 x 107 cells) and follow the manufacturer's instructions with 500 μ Clean each chromatographic column with PBS 0.5% FCS.
16. Add 250 μ L Cell suspension is loaded into each column. Magnetically labeled cells remain in the column, while unlabeled cells pass through. After the cell suspension flows through the column, use 500 μ PBS 0.5% FCS washing column twice. Please refer to the video or manual on the instrument manufacturer's website for instruments of different brands.
17. Remove the column from the magnet and use the provided plunger to elute the magnetically retained cells with PBS 0.5% FCS.
18. Wash the washed cells and centrifuge at 200 x g for 5 minutes. When laying the first board, the serum concentration of ECGM culture medium can be increased to about 10%, and then suspended in (10 ^ 5 cell/ml), And they were cultured in Petri dish or culture bottles pre coated with coating solution). Because the ECGM medium formula uses small molecular compounds to inhibit the growth of hybrid cells, and the use of protein to induce the growth of endothelial cells, the obtained endothelial cells can be more passaged and have higher purity. After 15 days of culture, no excessive growth of non endothelial source contaminated cells was observed at any stage. The purity of microvascular endothelial cells extracted and cultured by this method was more than 95%.







